
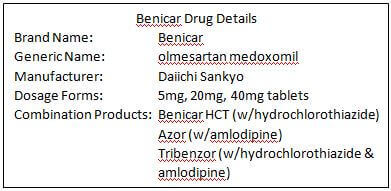
The Azor lawsuit said that neither von Eberstein, nor his doctor, knew of the link between the medication and GI health issues. Plaintiffs in Azor lawsuits share similar allegations, adding that the defendants knew or should have known that Azor use in humans was associated with and/or would cause intestinal problems. von Eberstein’s claim says that the Defendants “failed to adequately conduct complete and proper testing of Azor prior to and following the submission of its New Drug Application for Azor.” Can you buy Azor online approved canadian pharmacy kidney disease liver disease coronary artery disease angina (chest pain), congestive heart failure high levels of potassium in your blood if you are on a low-salt diet if you are 75 years or older or if you have recently had a heart attack. While the label was changed to reflect this information when the warning was announced, it was already too late for many patients. Not only were patients warned of the risk of “sprue-like enteropathy, including severe, chronic diarrhea with substantial weight loss and malnutrition in patients taking Azor,” but also that development of these problems could begin months to years after starting the drug. The FDA warned the public in 2013 about the dangerous gastrointestinal side effects tied to blood pressure medication Azor. What You Don’t Know Can Hurt YouĪzor (olmesartan/amlopidine) was linked to chronic diarrhea and weight loss by researchers at the Mayo Clinic in a study published in 2012. Our dental blog azor online is packed with important information that can benefit you and your whole family, ensuring that your next trip to one of our dental offices in Connecticut, Massachusetts, New Hampshire and New Jersey does not stretch your budget, consume your time or produce more aches and swelling. District Court for the Eastern District of Louisiana. The Azor side effects lawsuit against Daiichi Seiyaku, the manufacturer, accuses the company of failure to warn, defective design, breach of express warranty, and damages. The Azor label wasn’t updated until then, almost five years after von Eberstein started the medication.
According to his Azor lawsuit, von Eberstein “developed serious and dangerous side effects including sprue-like enteropathy, including severe, chronic diarrhea with substantial weight loss and malnutrition as well as other severe and personal injuries in which Plaintiff suffered physical pain and mental anguish, including diminished enjoyment of life, any and all life complications caused by intestinal problems, all past, present and future.”Īccording to the Azor lawsuit, von Eberstein did not know of the connection between the drug and his health complications until 2013, when the FDA informed the public that “use of the high blood pressure medication Azor may be associated with an increased risk of sprue-like enteropathy,” and went on to list the symptoms he was suffering. Soon thereafter his intestinal problems began.

About a year later, in late 2008, von Eberstein’s physician prescribed him Azor to combat his hypertension. Food and Drug Administration (FDA) in 2007, Azor was meant to treat high blood pressure. of Louisiana over Azor side effects.Īpproved by the U.S. A gastrointestinal side effects lawsuit was filed by plaintiff William H.


 0 kommentar(er)
0 kommentar(er)
